
The intriguing relationship between microbiota and human psyche showed gut microbiota to be associated with well-being. To determine if the microbiota of other body parts would be similarly associated, we analyzed the perceived stress, well-being, and happiness scale measures of 106 healthy individuals and their aerotolerant microbiota load in their otorhino canals. We found that both the perceived stress and well-being of the participants were associated with their otorhino microbial counts, but not their happiness scale scores. Women were found to have significantly less microbiota counts, lower well-being and higher levels of stress. This study is one of the first few to investigate new microbiota reservoirs in humans using aerobic body locations for psychobiological studies.
Stress is a complex process that involves environmental, biological, and psychosocial factors to initiate processes in both the central and peripheral nervous systems (Sapolsky, 1994). In this interaction, the biological aspect in the form of microorganisms in/on our body was found to play a possible role in stress management (Cryan and O’Mahony, 2011).
Microorganisms such as bacteria, fungi and even viruses, make up the microbiota in and on humans (Baron et al., 1994), sharing a symbiotic relationship. They often colonize humans at birth. From the very beginning of one’s life in passing through the birth canal, the human infant would acquire microbiota from the mother, and this acquisition process continues even when life ends to complete decomposition. While the microbiota was previously estimated to outnumber human cells by a ratio of 10:1 (Savage, 1977), recent studies have re-adjusted this ratio to be around 1.3:1 (Sender et al., 2016). Within the human body, these microorganisms can be found in a variety of places such as the ears, nose, mouth, gut and even on the skin (Baron et al., 1994), given that these body locations provide the necessary nutrients to promote and sustain the growth of the microbiota. In return for these nutrients, the microbiota helps to outcompete harmful pathogenic bacteria from colonization (Tancrede, 1992), and contribute functionally, such as in digestion (Macfarlane and Macfarlane, 2003), and modulating localized immune response (Macpherson and Harris, 2004).
Studies on the relationship between microbiota and behavior on male germ-free mice (mice grown in sterile environments) showed exaggerated amounts of stress hormones when the mice were subjected to mild restraint stress tests compared to control mice with normal microbiota composition and without prior exposure to specific pathogens (Sudo et al., 2004). These exaggerated stress hormones decreased when the germ-free mice were exposed to the fecal material of the control mice, with further reduction to normal ranges when fed with Bifidobacterium infantis (a common gut microbiota). The earlier the interventions were made, the greater the reversal of effects. When using female germ-free mice, the levels of gut microbiota were found to be correlated with reduced anxiety behavior and central neurochemical changes during the elevated plus maze (Neufeld et al., 2011). Since the administration of Lactobacillus rhamnosus was also found to reduce stress-induced symptoms in the mice, it was evident that the gut microbiota can have modulatory effects on animal stress, with possible gender differences (via biological hormones).
In humans, reduced microbial diversity in gut was found to be associated with disease, even pre-disease states. Obese individuals showed reduced bacterial diversity when compared to their leaner twin (Turnbaugh et al., 2009), and reduced microbiota diversity were also found in children with allergies (Y. Wang et al., 2009), rheumatoid arthritis (Vaahtovuo et al., 2008), and type 2 diabetes (Wu et al., 2010). Expectedly, the perturbation of the body microbiota can also affect stress responses (Rhee et al., 2009), particularly in examples where gastrointestinal disorders were reported with microbiota dysfunction in the co-morbidity of stress-related psychological disorders (Clemente et al., 2012). This relationship has been found to be so significant that the gut microbiota is now often called the “second brain” in the gut-brain axis (Ochoa-Reparaz and Kasper, 2016). This raises a possibility that besides the gut microbiota, other body areas may have such relationship with the psyche as well.
Despite these findings, research in this area is met with challenges in participant recruitment and sample collections which is, highly varied and difficult operationally. To make things worse, the research is often further hampered by time-consuming paperwork. Since human studies typically involve the anaerobic gut microbiota (bacteria to which oxygen is toxic), samples were collected either by the more invasive gut swabs or the contamination prone fecal sampling. Apart from being invasive to the participant and understandably unpleasant in working with human excrement, anaerobic culture methods require the tedious removal of oxygen from microbial culturing methods. As such, a less invasive and more pleasant sample collection alternative for humans is highly sought after.
Current literature on microbiota and psychological factors has two major shortcomings. Firstly, most of these studies were focused on animal studies with little validation in humans. While animals do exhibit similar chemical responses in stress such as the release of hormones, the difference in cognitive capabilities do not allow the same stress responses or management (Sapolsky, 1994). And the stress faced by modern humans such as those in the work place or in war, are often unique to the species. As such, the responses obtained from studies that focus purely on animals may not translate to humans. Secondly, the focus on the effect of gut microbes and their relationship with psychological factors, neglect the other areas of the human body that microbiota also flourish in, such as the ears and nose.
There are two general approaches to analyzing microbiota: diversity (types) and load/count. The complex nature of the host environment and the culture methods (e.g. aerobic/anaerobic) result in a great challenge to growing anaerobic gut microbiota that may result in biases. As such, opportunities to sample areas where the microbiota are aerobic or aerotolerant may allow for more accurate analysis. While microbiota diversity can be correlated to the well-being of the host organism (Bae et al., 2012; Frank et al., 2010), it is also highly variable. For example, studies conducted in Colorado suggested the nasal microbiota to consist primarily of Actinobacteria spp and other phyla such as Firmicutes spp and Proteobacteria spp (Frank et al., 2010), but other studies, e.g. in Korea, found the nasal microbiota to consist mainly of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and Staphylococcus aureus (Bae et al., 2012). To add on the complication, Staphylococcus aureus bacteria colonization was also reported to be negatively correlated with other microbial groups such as Staphylococcus epidermidis (Frank et al., 2010). Given the multiple confounding factors and natural complexity on microbiota diversity, and that current knowledge in this area remains insufficient, we sought a simpler sampling microbial analysis where instead of investigating diversity, we opted for microbial count alone as a biomarker. In doing this, we first sought to establish if there was a correlation between microbial load and the psychological parameters, and also if they contribute to well-being in relation to the hygiene hypothesis (a theory first reported by Strachan (1989) that the lack of early childhood exposure to microorganisms predisposes them to infections and immune diseases like allergies). The hygiene hypothesis is relevant here as it proposes a direct association for well-being (Renz et al., 2006) with respect to susceptibility of future infections, allergies (Wills-Karp et al., 2001; Yazdanbakhsh et al., 2002), and autoimmunity (Okada et al., 2010). In addition, the interest in microbial load over that of diversity is that diversity-based interventions are more complicated when compared to the load/count-based interventions, where there is no set standard to what constitutes as normal flora given that they can differ significantly from place to place, and also from region to region. In such a case, microbial load could be a more normalized criterion.
This study therefore aims to investigate: 1) the demographic factors of gender, religion, and ethnicity that may affect microbiota counts and the self-reported psychological measures; 2) the suitability of an aerobic/aerotolerant microbiota sampling method associated to self-reported levels of stress, well-being, and happiness in surveys.
With informed consent and ethics approval from James Cook University Human Research Ethics Committee (H6341), 134 participants aged 18-54 years old (M= 23.99 + 6.51) were recruited over a period of five months from the student population of James Cook University, Singapore, with the exclusion of those with recent oto (ear) –rhino (nose) -larynx (throat) infections or having taken antibiotics three months prior to the sample collection. Participants were briefed on the study and that their participation were voluntary, and they could withdraw without prejudice at any time during the sample collection. Student participants received university course credits for their participation, while non-student participants were volunteers without incentives. Using 3 predictors (power = .95, f2=.15), a priori computation analysis conducted using G*Power 3.1.9.2 for multiple regression tests suggested a minimum sample size of 119. Twenty-eight participants were removed due to the lack of any bacteria isolated from the swabs. Thus only 106 participants (65 male, 41 female) were analyzed. As the microbiological sampling method employed was non-invasive, and no human biological samples were collected/analyzed, but rather that of surface bacteria, there wereno further restrictions on our adopted sampling method.
Microbiota sampling took place in a quiet room where the participants used sterilized cotton buds (by autoclave involving high pressure and high heat steam) to swab their oral cavity on the inside of the cheeks and each of their otic and rhino canals ten times in each orifice. The cotton buds were then kept in phosphate buffer saline (PBS) in anonymized numbered tubes for later matching to the surveys. The participants next completed the Perceived Stress Scale (PSS) (Cohen et al., 1983), Depression Happiness Scale (DHS) (Joseph and Lewis, 1998), and Short Form-36 (SF-36) (McHorney et al., 1994) surveys. During which, the microbial swabs were serially diluted 10X and 100X with PBS before plating onto plate count agar (PCA) for common bacteria growth. The plates were incubated overnight at 37◦C and colony-forming unit/ml (cfu/ml) were counted. Statistical analyses including outlier, assumption testing and normality analyses were performed using IBM SPSS 24 (Statistical Package for Social Science).
Surveys used in the study included a demographics sheet (age, gender, nationality, and exclusion criteria of prior infections and antibiotic treatment within three months) and three sets of self-reported surveys. The surveys were: (i) perceived stress scale, (ii) depression happiness scale, and iii) the Short Form 36 which measures well-being.
Perceived Stressed Scale (PSS) measured the self-reported perceived stress (Cohen et al., 1983). The PSS was reported to have a Cronbach's α = .78 to .91 (Cohen and Janicki-Deverts, 2012) and contained ten items rated using a five-point Likert scale (0 = never to 4 = very often). Items 4, 5, 7, and 8 were scored in reverse order before the total sum of the scores were tabulated. The total possible scores ranged from 0 to 40, where higher scores indicated higher perceived stress experienced over the past one month. The PSS was favored over other stress measurement scales for its low question count and high reliability and validity (Roberti et al., 2006). Additionally, there was a clear cut-off point making it easier for data analysis. The Cronbach’s alpha for this scale was .87 in this study.
Depression Happiness Scale (DHS) is a 25-item self-report questionnaire developed by McGreal and Joseph (1993) that measures for happiness and depression. Participants reported how they felt in the past week and rated the frequency of each item on a 4-point scale: never (0), rarely (1), sometimes (2), and often (3). The DHS was utilized for its dual measurement of depression and happiness as opposite ends of the spectrum. Contrary to other more established measures of depressions such as Beck’s Depression Inventory (Beck and Beamesderfer, 1974), the measurement of both extremes of the emotion still allowed satisfactory reliability and validity (Joseph and Lewis, 1998) and possessed only 4 additional questions. The Cronbach’s alpha for this scale was .91 in this study.
The Short Form 36 (SF-36) is a 36-item scale developed by Ware & Sherbourne (1992) to assess the self-reported perceived health and quality of life in an individual. Through the rating of items on Likert-type scales ranging from 2-3 points to 5-6 points, the participant responded to 8 subscales: 1) limitations in physical activities due to health problems; 2) limitations in social activities due to physical or emotional problems; 3) limitations in usual role activities due to physical health problems; 4) bodily pain; 5) general mental health (psychological distress and well-being); 6) limitations in usual role activities due to emotional problems; 7) vitality (energy and fatigue); and 8) general health perceptions. SF-36 is also one of the most established scales for measuring perceived health with good reliability and validity (McHorney et al., 1993). In addition to its total score, the items in SF-36 can be divided into sub-scales allowing for deeper analyses. It should be noted that the subscales within the SF-36 do not possess the same psychometric strengths that the SF-36 possess and thus caution was placed for interpreting the results (Ware, 2000). The Cronbach’s alpha for this scale was .92 in this study.
The data were analyzed using the IBM Statistical Package for Social Science (SPSS) version 20.0. All tests conducted were based on an alpha value of .05. Prior to detailed analysis, inspections of normal probability plots and scatterplots of standardized residuals against standardized predicted values showed that assumptions of normality, linearity and homoscedasticity of residuals were met. High tolerances were found for all predictors within the regression model, indicating that multicollinearity would not interfere with the multiple regression analysis.
Although the oral microbiota samples of the participants were collected, the absence of colonies in aerobic conditions from most of the oral samples demonstrated inconsistency in the aerotolerant microbial diversity. This was not surprising since we employed aerobic conditions and that the oral microbiota can be often anaerobic (Sutter, 1984). Therefore, for consistency, we excluded oral microbiota from further analysis. The microbiota load was analyzed as the total otorhino count, but also included independent total otic and total nasal separately.
Using One-Way ANOVA (two-levels) and independent T-tests with gender as the factor, we found total otorhino microbiota count (as dependent variables) to be higher in males (~1.8 x 106 cfu/ml) than in females (~1.2 x 106 cfu/ml, F(1,104) = 2.941, p= 0.045, η2 = .027; t(104) = 1.718, p = 0.045, 1-tailed), with males having higher nasal microbiota (~0.6 x 106 cfu/ml) than females at ~0.3 x 106 cfu/ml, F(1,104) = 3.707, p = 0.029, η2 = .034; t(104) = 1.925, p = 0.029, 1-tailed. Women had higher PSS scores (19.85 + 6.26) than males (16.02 + 6.70), F(1,104)=8.683, p = 0.002, η2 = .077; t(104) = 2.947, p= 0.002, 1-tailed) with moderate effect size. Similarly, males reported higher DHS scores (74.86 + 11.05) than females (69.88 + 10.04) significantly at F(1,104) = 5.480, p = 0.011, η2 = .050; t(104) = 2.341, p = 0.011, 1-tailed) with moderate effects, as well as for psychological well-being (~7342.41 + 15.41 vs 69.41 + 15.74, F(1,104) = 4.831, p = 0.015, η2 = .044; t(104) = 2.198, p=0.015, 1-tailed) with moderate effects. There were no significant (p >0.05) gender differences found for total SF-36 scores and its sub-scales (limitations in physical, usual roles, pain, health perception, and emotional issues), as well as for otic microbiota counts. 1-tailed experiments were adopted as we expected males to exhibit higher microbial counts due to increased secretions from general higher metabolic rates.
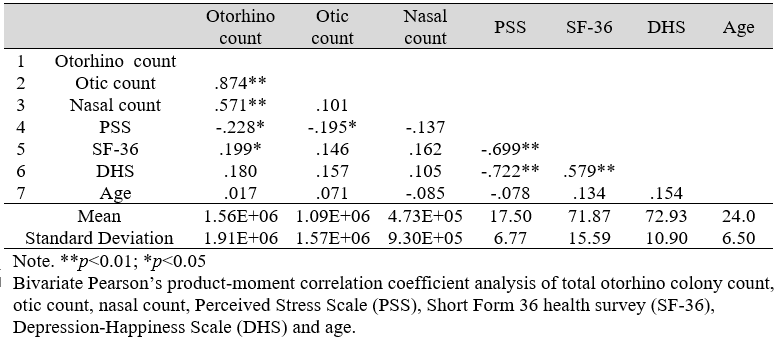
Table 1: Mean, Standard Deviation, and Correlations among variables (N=106)
Based on one-way ANOVA (two-levels), there were no significant differences found in all tested parameters (psychological and microbial) for ethnicity and religious affiliations. For nationality, Singaporeans reported lower well-being (DV being SF-36 scores of 70.35 + 15.81 vs 79.85 + 11.78) than non-Singaporeans (F (1,104) = 5.528, p=0.021, η2 = .050) with moderate effects. However, non-Singaporeans reported significantly higher limitations due to emotional problems (SF-36 sub-scale scores of 86.27 + 29.01 vs 68.16 + 38.24) and limitations in social activities due to physical or emotional problems (SF-36 subscales scores of 86.03 + 16.47 vs 73.60 + 22.41) significantly at F (1,104) = 3.425, p= 0.034, η2 = .032 and F (1,104) = 4.728, p = 0.016, η2 = .043 (1-tailed) with small and moderate effects, respectively.
Using linear regression, we did not find any correlations between age and total microbial count, perceived stress, well-being, and happiness.
Bivariate Pearson’s correlation analysis of nasal, otic, total otorhino microbial count, age, PSS, SF-36, and DHS (Table 1) revealed significant correlations between otic microbial count and PSS; total microbial count and both PSS and SF-36; PSS and both SF-36 and DHS; and SF-36 with DHS. Expectedly, both otic and nasal counts were correlated to total otorhino microbial count.
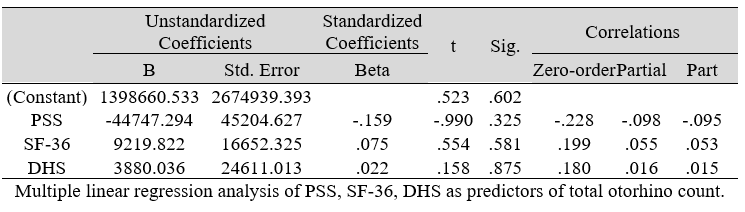
Table 2: Multiple Regression Analysis of the predictors of Microbial Count (DV)
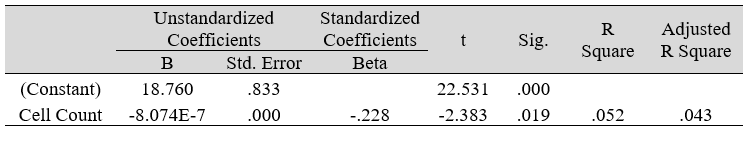
Table 3A: Regression analysis between PSS and predictor: total otorhino microbial count (DV)
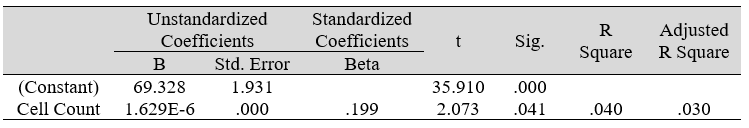
Table 3B: Regression analysis between SF-36 and predictor: total otorhino microbial count (IV)
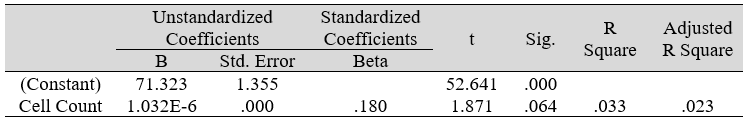
Table 3C: Regression analysis between DHS and predictor: total otorhino microbial count (IV)
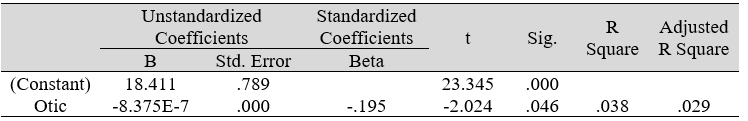
Table 3D: Regression analysis between PSS and predictor: otic microbial count (IV)
Multiple regression analysis of total nasal only, total otic only, and total otorhino microbiota count showed no significant effects on stress, well-being and happiness. Further analyses suggested that a large portion of the variance was shared among all three independent variables (IVs) of PSS, SF-36 and DHS (“zero-order” was higher than the “partial” or “part” values, see Table 2). Total otorhino microbial count showed a significant regression equation with perceived stress (Table 3A) and well-being (Table 3B) as well as with DHS (Table 3C). Individual regressions of the otic and nasal counts as co-factors with PSS, SF-36, and DHS, did not yield significant regression equations (Table 3E, F, G). When analyzed independently, with the exception of otic alone with PSS (Table 3D), total otic or total nasal counts also did not yield statistically significant (p >0.05) equations. This suggests that by itself, the otic or nasal count alone were insufficient for statistical significance, and analyses would benefit from a total count of both areas.
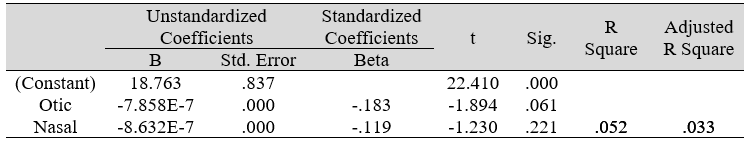
Table 3E: Regression analysis between PSS and predictors: otic and nasal microbial count
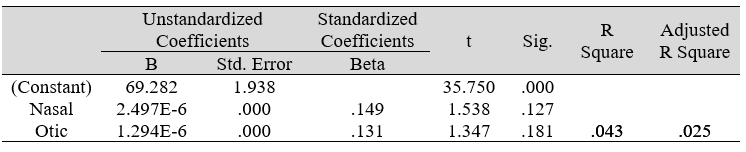
Table 3F: Regression analysis between SF-36 and predictors: otic and nasal microbial count
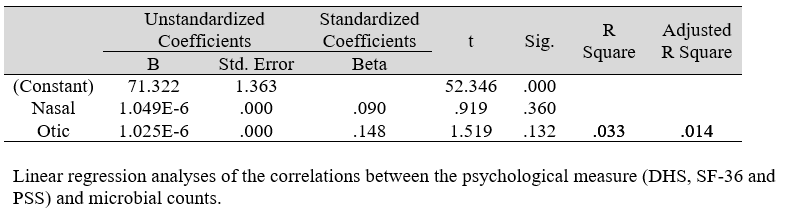
Table 3G: Regression analysis between DHS and predictors: otic and nasal microbial count
We set out to investigate the relationship between the self-reported psychological measures of stress, general well-being, and happiness from surveys with the otorhino aerobic/aerotolerant microbial count in healthy human participants. This study is the first of its kind to utilize a more researcher and participant friendly sample collection method. Given the aerobic/aerotolertant nature of otorhino microbiota when compared to the anaerobic makeup of the gut microbiota, culture conditions were significantly less invasive, pleasant, and more convenient for research. Although we had collected oral microbiota, we found it to vary significantly from individual to individual where the majority of the samples collected had no colony growth in our aerobic conditions. This was expected given the anaerobic nature of the oral microbiota (Sutter, 1984), and thus our analyses were restricted only to the microbial load of the otorhino cavities.
In our collection of microbiota samples, we found 28 participants to have no microbial growth in all their cultures. Possibilities for this can include the fastidious nature of the microbiota to non-compliance to the sampling instructions. As such, the data from these participants were excluded. Nonetheless, this was less of a problem compared to a previous study where 46% of their cultures yielded no growth, even when taken by medical professionals (Rusan et al., 2009). While pre-admission antibiotics were likely the major contributing factor for their study, it was unlikely to account for such high numbers of no growth. Regardless, both participants and researchers preferred to have the participants sample themselves, and our lower percentage of no growth at ~21% support our collection method.
With the exception that otic microbiota was inversely correlated with perceived stress, our demographic analysis of the nasal and otic microbiota separately did not yield any notable associations with the psychological measures. Such findings push for further studies to be inclusive of both otic and rhino counts rather than any one area alone given that statistically, more sampling areas would better represent the body microbiota. In our gender analysis, males had higher nasal microbiota count than females (but not for total otorhino or otic count alone), reported lower stress levels, higher happiness, and higher psychological well-being. While the reasons for such observations may be mediated by sex hormones (e.g. gender effects on microbial load as suggested by Yurkovetskiy et al. 2013), we acknowledge that there could be other socio-economic-political factors that are beyond the scope of this study, and it would too speculative to discuss further on this.
We did not find microbial load or the other psychological factors (stress, well-being, and happiness) to be affected by age although a previous study reported such correlations for microbial diversity (Hopskins et al., 2001). Our results suggest that microbial load was more consistent than microbial diversity, thus making it more reliable a parameter for such studies. Nonetheless, microbial load by itself does have limited biological significance, as a high microbial load does not provide information on levels of harmful or beneficial bacteria which would clearly make a difference. Yet, for direct simple measurements, microbial load does not incur high biomedical research costs typical of microbial identification experiments.
In our study, Singaporeans reported lower well-being than non-Singaporeans. However, the latter reported more constraints due to emotional and social activity limitations, which can be expected for non-natives in a foreign land.
The same lack of significant effects by religious affiliation and ethnicity was also prevalent in our study, demonstrating that our sample population was relatively homogenous on the psychological measures and microbiota despite these differences.
When analyzing the microbiota load, our results were contrary to previous experiments on animals (Bravo et al., 2011) and humans (Rao et al., 2009) where we did not find happiness to correlate to microbiota. Such discrepancies may be due to different sampling locations or diversity, and by the fact that happiness is often influenced by culture, religions, amongst other socio-economic factors.
Regardless of the finding on happiness from the DHS scale, we found inverse correlation for microbiota counts to perceived stress and well-being (perhaps lending limited support to the hygiene hypothesis that excessive cleanliness may not be good for health). As a between-subjects study, we were not able to determine causality effects, and the exact mechanisms and even the direction for the relationship remain enigmatic. Between stress, well-being, and otorhino microbiota, the cause and effect or even mediation effects would be at best, speculative.
On the causative relationship between stress and microbiota, research have suggested that bacteria can mitigate stress responses (Neufeld et al., 2011; Sudo et al., 2004) via nerves. A study using Salmonella Typhimurium bacteria found upregulated c-fos gene expression within the paraventricular and supraoptic nucleus of the hypothalamus of mice after intubation of the bacteria (Wang et al., 2002). The effects were ablated with a vagotomy (severing of the vagus nerve), as also shown in probiotic experiments (Bravo et al., 2011).
On the other hand, other studies have suggested stress to be the mitigating factor on microbiota. In one such proposal was that stress-induced hyperthermia (Oka, 2015) observed in rats (Zethof et al., 1995), pigs (Parrott et al., 1995), rabbits (Frosini et al., 2003), and humans (Briese, 1995) could affect the microbiota (Pickering, 1958). Similarly, stress-induced increased permeability of mucosal membranes mediated by stress hormones, e.g. hormone corticotropin-releasing factor (CRF) (Bloom et al., 1982), also antagonized microbial attachment (Barbara et al., 2005; Huycke and Gaskins, 2004). In fact, the gut permeability changes were found to be three-fold twelve days after CRF administration (Teitelbaum et al., 2008), proposing an alternative explanation to why stressed individuals had reduced microbiota. This may explain our findings on perceived stress since the otorhino canals are also mucosal membranes.
Current literature in the field remains uncertain to the exact direction of microbiota-stress relationship. Nonetheless, as in bodily physiological responses, there is likely to be a homeostatic feedback mechanism involved, where one parameter (stress or microbiota) would likely affect the other in a balancing relationship. By manipulating this balance, it may be possible to modify microbiota load or diversity as an intervention for stress management. Nonetheless, there is a clear dearth of research in this area, on humans. While our study demonstrated an inverse correlation between the microbiota counts with stress and well-being, the results were not completely unexpected given the earlier studies in germ-free mice exhibiting more stress and anxiety in tests. There is still much to be investigated, particularly on diversity (if research funds and timespans allow), more aerobic areas (to include skin swabs of certain regions), and a control against the presence of anaerobic bacteria together.
Our pioneering “Psycho-otorhinomicrobiology” study found the less-invasive nose and ear microbiota sampling methods to be more pleasant for research, where this sample collection method could be used for future research without incurring high research costs, long research timespan, and also reducing ethical application concerns. While we acknowledge that there could be inter-participant variations in their levels of comfort on scraping their facial canals and their own self-biases in the self-reported surveys, such sampling methods may be the best way forward. Even our findings for the oral microbiota, which was eventually dropped, demonstrated the challenges involved in sampling anaerobic regions. With improved methods and future research, the advent of “Psycho-otorhinomicrobiology” in the complex interaction between brain, mind, and physiology might be just around the corner.
This work was partially supported by JCO1334i00050 from the Joint Council Office, Agency for Science, Technology, and Research, Singapore. Ethics approval was obtained from James Cook University Human Research Ethics Committee (H6341) with informed consent by the participants.
The work described here was the final year project of BYLG in fulfilment of the requirements of the Bachelor in Psychology from James Cook University, Singapore.
SG is also the Editor-In-Chief of the journal. To avoid conflict of interest, the article was handled by an independent member of the editorial board. The decision for the manuscript was fast-tracked based on previous reviews for the article by previously submitted journals. The article-processing-charge for this article was waived under quota for Bioinformatics Institute, A*STAR.
BYLG performed the experiments, carried out the psychological surveys, and drafted the manuscript. JYY performed part of the statistics as well as formatted the manuscript. SKEG conceived the idea, designed the experiments, and supervised all aspects of the project and writing. All authors read and approved the final manuscript.
Bae, S., Yu, J. Y., Lee, K., Lee, S., Park, B., and Kang, Y. (2012). Nasal colonization by four potential respiratory bacteria in healthy children attending kindergarten or elementary school in Seoul, Korea. J Med Microbiol, 61, 678-685. doi:10.1099/jmm.0.040584-0
Barbara, G., Stanghellini, V., Brandi, G., Cremon, C., Di Nardo, G., De Giorgio, R., and Corinaldesi, R. (2005). Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol, 100(11), 2560-2568. doi:10.1111/j.1572-0241.2005.00230.x
Baron, E. J., Chang, R. S., Howard, D. H., Miller, J. N., and Turner, J. A. (1994). Medical Microbiology: A short course. New York: Wiley-Liss.
Beck, A. T., and Beamesderfer, A. (1974). Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry, 7(0), 151-169.
Bloom, F. E., Battenberg, E. L., Rivier, J., and Vale, W. (1982). Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regul Pept, 4(1), 43-48.
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., Bienenstock, J., and Cryan, J. F. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. PNAS, 108(38), 16050-16055. doi:10.1073/pnas.1102999108
Briese, E. (1995). Emotional hyperthermia and performance in humans. Physiol Behav, 58(3), 615-618.
Clemente, J. C., Ursell, L. K., Parfrey, L. W., and Knight, R. (2012). The impact of the gut microbiota on human health: an integrative view. Cell, 148(6), 1258-1270. doi:10.1016/j.cell.2012.01.035
Cohen, S., and Janicki-Deverts, D. (2012). Who's Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 2009. J Applied Soc Psy, 42(6), 1320-1334. doi:10.1111/j.1559-1816.2012.00900.x
Cohen, S., Kamarck, T., and Mermelstein, R. (1983). A global measure of perceived stress. J Health Soc Behav, 24(4), 385-396.
Cryan, J. F., and O’Mahony, S. M. (2011). The microbiome‐gut‐brain axis: from bowel to behavior. Neurogastroenterol Motil, 23(3), 187-192. doi:10.1111/j.1365-2982.2010.01664.x
Frank, D. N., Feazel, L. M., Bessesen, M. T., Price, C. S., Janoff, E. N., and Pace, N. R. (2010). The human nasal microbiota and Staphylococcus aureus carriage. PLoS ONE. doi:journal.pone.0010598
Frosini, M., Sesti, C., Saponara, S., Ricci, L., Valoti, M., Palmi, M., Machetti, F., and Sgaragli, G. (2003). A specific taurine recognition site in the rabbit brain is responsible for taurine effects on thermoregulation. Br J Pharmacol, 139(3), 487-494. doi:10.1038/sj.bjp.0705274
Hopskins, M., Sharp, R., and Macfarlane, G. (2001). Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut, 48(2), 198-205. doi:10.1136/gut.48.2.198
Huycke, M. M., and Gaskins, H. R. (2004). Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp Biol Med (Maywood), 229(7), 586-597.
Joseph, S., and Lewis, C. A. (1998). The Depression–Happiness Scale: Reliability and validity of a bipolar self-report scale. J Clin Psychol, 54(4), 537-544.
Macfarlane, S., and Macfarlane, G. T. (2003). Regulation of short-chain fatty acid production. Proc Nutr Soc, 62(1), 67-72. doi:10.1079/PNS2002207
Macpherson, A. J., and Harris, N. L. (2004). Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol, 4(6), 478-485. doi:10.1038/nri1373
McHorney, C. A., Ware, J. E. J., Lu, J. F., and Sherbourne, C. D. (1994). The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care, 32(1), 40-66.
McHorney, C. A., Ware, J. E. J., and Raczek, A. E. (1993). The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care, 31(3), 247-263.
Neufeld, K. M., Kang, N., Bienenstock, J., and Foster, J. A. (2011). Reduced anxiety‐like behavior and central neurochemical change in germ‐free mice. Neurogastroenterol Motil, 23(3), 255-264, e119. doi:10.1111/j.1365-2982.2010.01620.x
Ochoa-Reparaz, J., and Kasper, L. H. (2016). The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr Obes Rep, 5(1), 51-64. doi:10.1007/s13679-016-0191-1
Oka, T. (2015). Psychogenic fever: how psychological stress affects body temperature in the clinical population. Temperature (Austin), 2(3), 368-378. doi:10.1080/23328940.2015.105690
Okada, H., Kuhn, C., Feillet, H., and Bach, J.-F. (2010). The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol., 160(1), 1-9. doi:10.1111/j.1365-2249.2010.04139.x
Parrott, R. F., Vellucci, S. V., Forsling, M. L., and Goode, J. A. (1995). Hyperthermic and endocrine effects of intravenous prostaglandin administration in the pig. Domest Anim Endocrinol, 12(2), 197-205.
Pickering, S. G. (1958). Regulation of body temperature in health and disease. The Lancet, 271(7011), 59-64. doi:10.1016/S0140-6736(58)92564-9
Rao, A. V., Bested, A. C., Beaulne, T. M., Katzman, M. A., Iorio, C., Berardi, J. M., and Logan, A. C. (2009). A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog, 1, 6. doi:10.1186/1757-4749-1-6
Renz, H., Blümer, N., Virna, S., Sel, S., and Garn, H. (2006). The immunological basis of the hygiene hypothesis. Chem Immunol Allergy, 91, 30-48. doi:10.1159/000090228
Rhee, S. H., Pothoulakis, C., and Mayer, E. A. (2009). Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol, 6(5), 306-314. doi:10.1038/nrgastro.2009.35
Roberti, J. W., Harrington, L. N., and Storch, E. A. (2006). Further psychometric support for the 10‐item version of the perceived stress scale. J College Counseling, 9(2), 135-147. doi:10.1002/j.2161-1882.2006.tb00100.x
Rusan, M., Klug, T. E., and Ovesen, T. (2009). An overview of the microbiology of acute ear, nose and throat infections requiring hospitalisation. Eur J Clin Microbiol Dis, 28(3), 243-251. doi:10.1007/s10096-008-0619-y
Sapolsky, R. M. (1994). Why Zebras Don’t Get Ulcers: A Guide to Stress, Stress-Related Diseases, and Coping. New York: W. H. Freeman.
Savage, D. C. (1977). Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol, 31, 107-133. doi:10.1146/annurev.mi.31.100177.000543
Sender, R., Fuchs, S., and Milo, R. (2016). Revised estimates for the number of human and bacteria cells in the body. Plos Biol, 14(8), e1002533. doi:10.1371/journal.pbio.1002533
Strachan, D. P. (1989). Hay fever, hygiene, and household size. BMJ, 299(6710), 1259-1260.
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X. N., Kubo, C., and Koga, Y. (2004). Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol, 558, 263-275.
Sutter, V. L. (1984). Anaerobes as normal oral flora. Rev Infect Dis, 6, S62-66.
Tancrede, C. (1992). Role of human microflora in health and disease. Eur J Clin Microbiol Infect Dis, 11(11), 1012-1015.
Teitelbaum, A. A., Gareau, M. G., Jury, J., Yang, P. C., and Perdue, M. H. (2008). Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. Am J Physiol Gastrointest Liver Physiol, 295(3), G452-459. doi:10.1152/ajpgi.90210.2008
Turnbaugh, P. J., Hamady, M., Yatsunenko, T., Cantarel, B. L., Duncan, A., Ley, R. E., Sogin, M. L., Jones, W. J., Roe, B. A., Affourtit, J. P., Egholm, M., Henrissat, B., Heath, A. C., Knight, R., and Gordon, J. I. (2009). A core gut microbiota in obese and lean twins. Nature, 457, 480-484.
Vaahtovuo, J., Munukka, E., Korkeamäki, M., Luukkainen, R., and Toivanen, P. (2008). Fecal microbiota in early rheumatoid arthritis. J Rheumatol, 35(8), 1500-1505.
Wang, X., Wang, B. R., Zhang, X. J., Xu, Z., Ding, Y. Q., and Ju, G. (2002). Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J Gastroenterol, 8(3), 540-545.
Wang, Y., Hoenig, J. D., Malin, K. J., Qamar, S., Petrof, E. O., Sun, J., Antonopoulos, D. A., Chang, E. B., and Claud, E. C. (2009). 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J, 3(8), 944-954. doi:10.1038/ismej.2009.37
Ware, J. E. J. (2000). SF-36 health survey update. Spine (Phila Pa 1976), 25(24), 3130-3139.
Wills-Karp, M., Santeliz, J., and Karp, C. L. (2001). The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol, 1(1), 69-75. doi:10.1038/35095579
Wu, X., Ma, C., Han, L., Nawaz, M., Gao, F., Zhang, X., Yu, P., Zhao, C., Li, L., Zhou, A., Wang, J., Moore, J. E., Millar, B. C., and Xu, J. (2010). Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol, 61(1), 69-78. doi:10.1007/s00284-010-9582-9
Yazdanbakhsh, M., Kremsner, P. G., and van Ree, R. (2002). Allergy, parasites, and the hygiene hypothesis. Science, 296(5567), 490-494. doi:10.1126/science.296.5567.490
Yurkovetskiy, L., Burrows, M., Khan, A. A., Graham, L., Volchkov, P., Becker, L., Antonopoulos, D., Umesaki, Y., and Chervonsky, A. V. (2013). Gender bias in autoimmunity is influenced by microbiota. Immunity, 39(2). doi:10.1016/j.immuni.2013.08.013
Zethof, T. J., Van der Heyden, J. A., Tolboom, J. T., and Olivier, B. (1995). Stress-induced hyperthermia as a putative anxiety model. Eur J Pharmacol, 294(1), 125-135.